The current Adverse reaction risk archetype was first published in March 2016 after a lengthy modelling and review process detailed in this blog post by @heather.leslie.
The Adverse reaction risk archetype was always intended to be used for recording the positive presence of a general propensity to react badly to specific substances or groups of substances, for the purpose of alerting clinicians to avoid or be careful when exposing the patient to said substances. An outstanding issue has been how to record information about specific reactions when they occur out of the blue, or in a situation when you expect that a reaction is more likely to occur, such as in the first 30 minutes after vaccination.
Recently these requirements have showed up in actual implementation projects, which has reinvigorated modelling efforts in this area as well as provided use cases to guide modelling patterns.
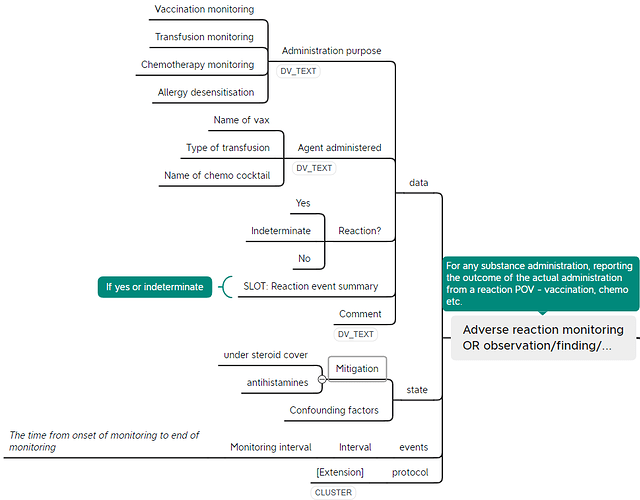
Based on the use cases of monitoring a patient for adverse reactions after such interventions as vaccination, hyposensitisation, transfusion or chemotherapy, we’re proposing a new OBSERVATION archetype with the tentative name of ‘Adverse reaction monitoring’ (see preliminary mindmap below). As part of the scoping out of this area, it was identified that the internal cluster ‘Reaction event’ currently in the Adverse reaction risk archetype would be useful in both of these two ENTRY archetypes. We’re therefore proposing to separate this out as a CLUSTER archetype, which would mean a breaking change to the Adverse reaction risk archetype.
Additionally, we’re looking at how to best represent provocation tests such as skin prick tests, mantoux, food item provocation, etc. We’ve also briefly discussed food elimination tests as a separate concept in this space.